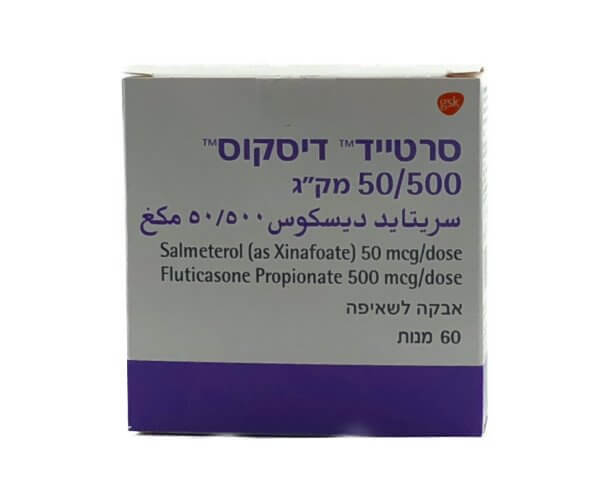
Advair®/Seretide® includes fluticasone, a steroid that prevents inflammation, and salmeterol, a bronchodilator that relaxes muscles in the bronchial tubes to assist with breathing. Advair®/Seretide® uses these drugs to help to prevent asthma attacks and treat chronic obstructive pulmonary disease (COPD), which is associated with chronic bronchitis.
Seretide HFA strength is equivalent to Advair HFA as follows:
Seretide HFA 125/25mcg = Advair HFA 115/21mcg
Seretide HFA 250/25mcg = Advair HFA 230/21mcg
Brand Manufacturer:
Side Effects: Upper respiratory tract infections occur in 20% to 25% of patients using Advair. Headaches occur in about 1 in 8 patients who use it. Other potential adverse events include nausea, vomiting, diarrhea, mouth or throat candidiasis, and musculoskeletal pain. Use of long acting agents like salmeterol, an active ingredient in Advair, may increase the risk of asthma-related death. Therefore, Advair should only be used in patients who’s asthma is uncontrolled by other agents, and who are using long-term asthma-controlling medications such as an inhaled corticosteroid.
Indication: Advair Diskus is used for the treatment of asthma or chronic obstructive pulmonary disease (COPD) associated with chronic bronchitis. Its action starts within 30 to 60 minutes and can last more than 12 hours. Advair Diskus is generally not needed in patients whose asthma can be controlled easily with infrequent administration of short acting inhalers. Advair HFA is used for treating asthma in individuals 12 years old or older. Advair Diskus or HFA should not be used to treat acute episodes of asthma or COPD.
Reviews
Write a review
The product has no reviews
Leave your review